Portfolio overview
The Ziccum pipeline of external projects is depicted in a portfolio overview. This gives a general representation of the key steps towards the desired commercialization by entering into license agreements, licensing the LaminarPace technology for specific applications, and the current status of each project. The actual progress in a specific project may proceed via alternative or additional steps, and the timeline varies greatly depending on the resulting read-outs and the counterpart preferences.
Pharmaceutical development in general is subject to very strict confidentiality, and certain collaborations are given without partner name publication, until name disclosure is possible.
The company also pursues earlier dialogues with other counterparts in on-going business development efforts.
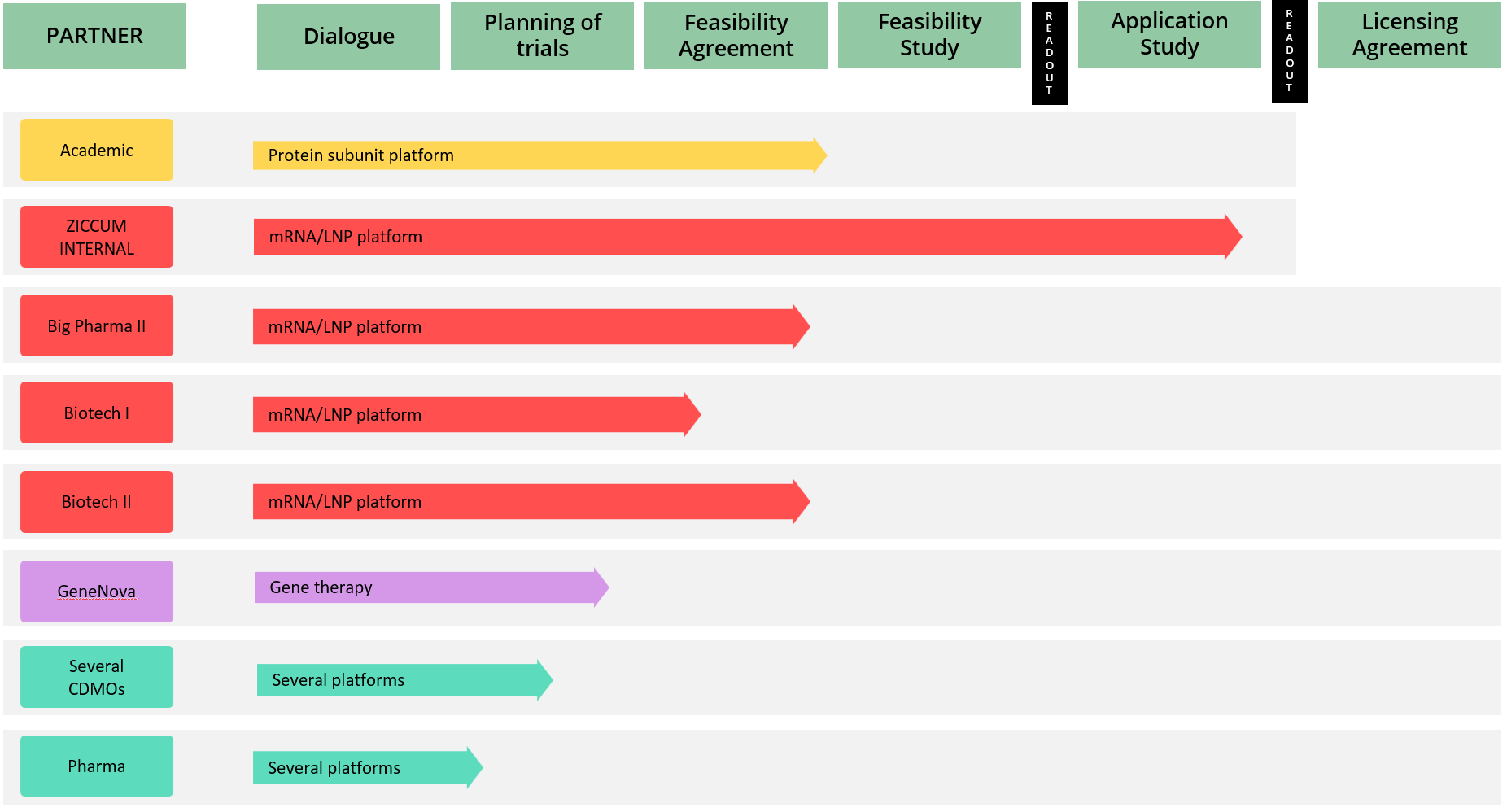
Project portfolio overview as of 31 March, 2023
*The text in the arrow represents the technology platform
Ziccum in brief
Ziccum is developing LaminarPace, a unique ambient drying method for biopharmaceuticals and vaccines based on mass transfer, not heat transfer. The technology is offered by licensing to vaccine and biologics developers and manufacturers in the global pharmaceutical industry.
By reducing drying stress to the active ingredient, LaminarPace uniquely enables particle-engineered, thermostable dry powder biopharmaceuticals which can be easily handled and transported and are highly suitable for novel administration routes. The technology has been successfully applied to mRNA, peptides, proteins, antibodies, lipids and enzymes as well as excipients and adjuvants, and is well suited for industrial application.

