Expected future development
The company's overall objective is to enter into license agreements to industrialize and commercialize the technology in collaboration with one or more major pharmaceutical companies.
The path to licensing agreements goes through evaluation agreements where LaminarPace functionality and capacity are evaluated together with a partner. If successful, the ambition is to continue to a negotiation regarding a license agreement. Primarily for a specific project or vaccine.
A prerequisite for being a relevant and attractive licensing partner is to be able to describe what an industrial version of LaminarPace can look like, and make it probable that the technology is suitable for upscaling and GMP production. Therefore, Ziccum conducts its own development projects where important components in LaminarPace are developed and adapted to industrial requirements. Ziccum is carrying out intensive work on developing 3D modelling, and ultimately a Digital Twin, of LaminarPace in partnership with the ICP Institute of Computational Physics team at the Zurich University of Applied Sciences’s School of Engineering (ZHAW.) The 3D modelling is being used to optimize LaminarPace design, exploring optimal capacity loads and increasing the repeatability of outcomes. It will be a valuable enabler of tech transfer and integration into existing pharmaceutical production chains.
Another priority area is applications for external and non-dilutive funding for further development of the technology. Ziccum actively monitors announcements that suit the Company's area of operation and technical phase.
Project Portfolio overview
The Ziccum pipeline of external projects is depicted in a portfolio overview. This gives a general representation of the key steps towards the desired commercialization by entering into license agreements, licensing the LaminarPace technology for specific applications, and the current status of each project. The actual progress in a specific project may proceed via alternative or additional steps, and the timeline varies greatly depending on the resulting read-outs and the counterpart preferences.
Pharmaceutical development in general is subject to very strict confidentiality, and certain collaborations are given without partner name publication, until name disclosure is possible.
The company also pursues earlier dialogues with other counterparts in on-going business development efforts.
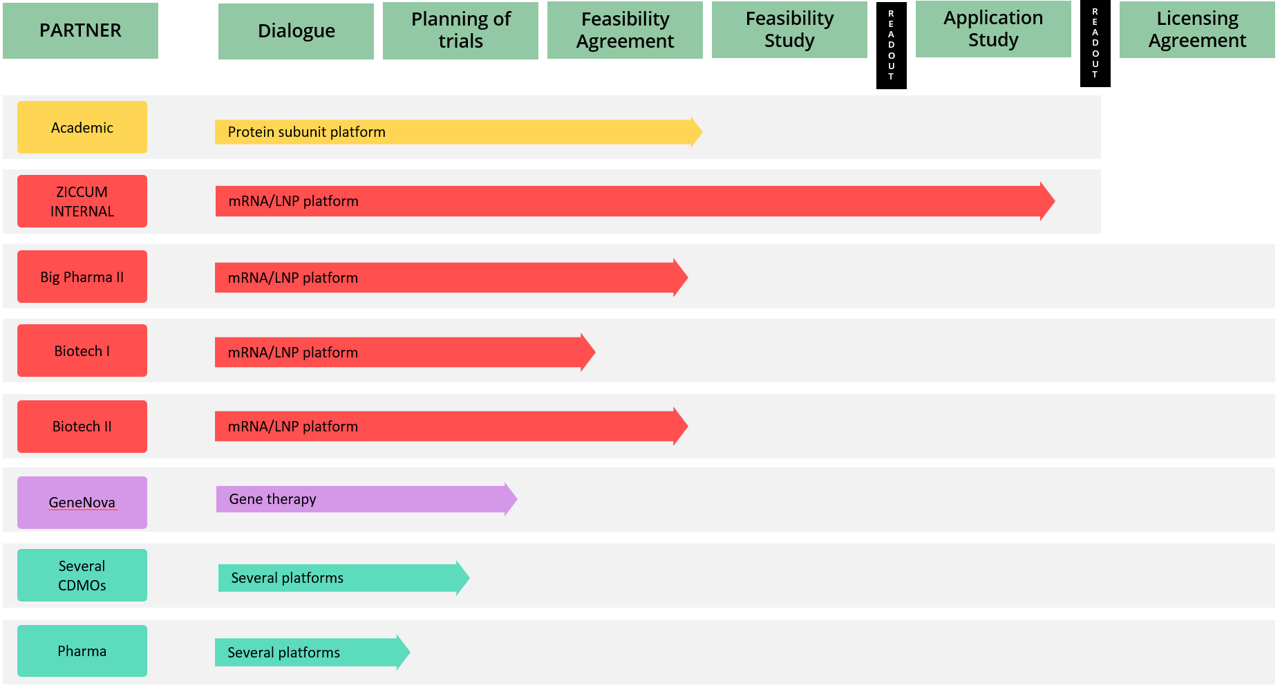
Project portfolio overview as of 31 March, 2023
*The text in the arrow represents the technology platform